Rethinking Cleanroom Design
The new ISO 14644-4 was published in November 2022 and addresses design, construction, and start-up of Cleanrooms. But why has the Air Change Rate table from Annex B.2 disappeared?
Well, because no one size fits all; the old table led to over-design in many cases and a lack of focus on understanding the process and contamination risks. The new and revised Annex 1 places the focus on QRM (Quality Risk Management) and CCS (Contamination Control Strategy) instead.
The ISO/TC 209 working group, composed of subject matter experts from nearly 20 countries (including Ireland, UK, France, Germany, Switzerland, The Netherlands, Italy, Sweden, Norway, Denmark, Romania, Russia, China, Japan, Australia, Philippines, Korea, Brazil, and the USA) decided there was a better way – good engineering practice (GEP) and good science would prevail!
The Evolution of ISO 14644 Standards
Back in the late 1990s when the first version (2001) was being developed the experts at the time agreed, that as part of the “first generation” of the new ISO 14644 standard series of dashes, they would include some general guidance. They put the Air Change Rate table in the Informative Annex purely as a backup plan, to be used in the event of no other information being available. Unfortunately, lots of people, both users and designers adopted the Air Change Rate table, which was often cross referenced in the URS. The need for compliance in a heavily regulated industry paved the way for this approach and this became the norm over time.
To make matters worse, from a Life Science perspective the language and guidance was driven by the Microelectronics Industry, which at the time was operating at very low particle thresholds and was heavily reliant on unidirectional airflows and high concentrations of HEPA filtered air. So, when we look at ISO 7 and IOS 8 in the context of Life Science and Sterile Medicines in Annex 1, terms like “Service Zones” and “Surface Treatment” meant nothing.
Guide to Good Manufacturing Practice
The rules governing medicinal products in the European Union were issued by the EU around the same time as ISO 14644 was first issued, and EU Pharmacopoeia became the benchmark for the manufacture of medicinal products.
The directive laying down principles and guidelines of good manufacturing practice (GMP) for medicinal products for human use (Directive 91/356/EEC) was adopted by the Commission in 1991. Detailed guidelines in accordance with those principles are published in the Guide to Good Manufacturing Practice (EudraLex) which is used in assessing applications for Manufacturing Authorisations and as a basis for inspection of manufacturers of medicinal products by the Regulator.
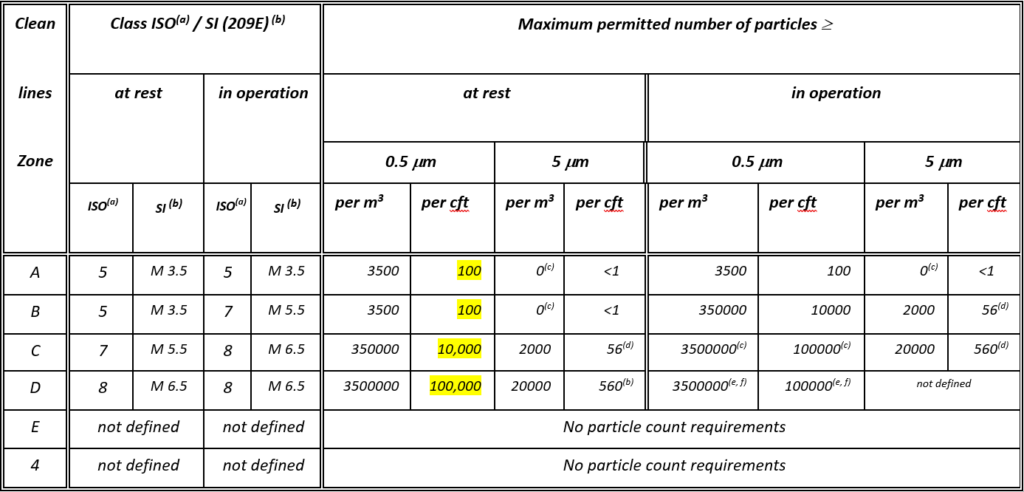
For the manufacture of sterile medicinal products there are 4 grades of clean ware specified
Areas, Grades A, B, C, & D.
In the original ISO 14644-4:2001 the guidance given for the maximum permitted number of particles in the “At Rest” condition corresponded approximately to the US Federal Standard 209E and the ISO 14644 classifications as follows:
Grades A and B correspond with class 100, M 3.5, ISO5
Grade C with class 10.000, M 5.5, ISO 7
Grade D with class 100.000, M6.5, ISO 8
Hence Grade A, B, C and D facilities for the manufacture of medicinal products became inextricably linked to ISO 14644 cleanroom classifications and unfortunately tagged with ACR (Air Change Rates).
Table of Comparison of ISO 14644-4:2001 and ISO 14644-1:1999 vs FED STD 209E & Annex 1 (1971 1st version)
The original ISO 14644-4:2001 very much derived from the Microelectronics and Space industries. In the Life Science and Pharma Industries, terminology for ISO 5 was never aligned to Grade A applications, where the terms used included “multi-layer processing” and “compact discs”. However, the biggest misuse came from “Note D” where the tables related to a nominal ceiling of 3m.
It’s possible the Air Change Rate table has been quoted and misused for over 2 decades.
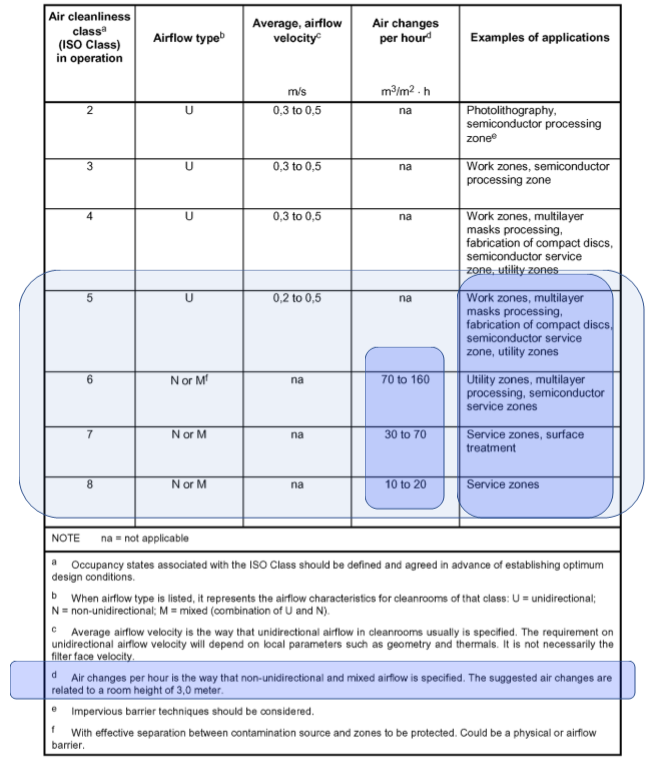
Examples of microelectronic cleanrooms
Expanding the Scope in the new ISO 14644-4:2022
When ISO/TC 209 set out to revise the 14644-4 standard it was clear it needed to address the needs of Life Science applications and not just Microelectronics and Semiconductors.
This time, the experts were aware of the EMA and PIC/S joint IWG (Inspectors Working Group) effort in revising the 2008 version of Annex 1.
The ISO/TC 209 working group of international experts went back to good engineering practice. It has been clear to Cleanroom experts for many decades that unidirectional flow, UDAF (used to be called “Laminar Flow”) could be used to effectively flush critical areas, such as points of fill with HEPA or, more commonly now, ULPA filtered air to create sterile conditions. The almost wholesale adoption of RABS and Isolators for Grade A conditions is evidence of this, never mind the fact that this removes a lot of human intervention. The current trend is towards Robotic and “gloveless” isolators and RABS. The greatest effect on the particle concentration in a non-unidirectional airflow (non-UDAF), or Turbulent/Dilution flow cleanroom comes from the dynamic relationship between what particles are generated within the cleanroom, and the manner and mechanisms in which they are removed.
Key Takeaway
When HEPA filters are used the dominant source of contamination comes from traffic, i.e., what comes into the cleanroom plus what is generated and associated with the process inside the cleanroom, i.e., equipment, materials, personnel consumables, and other work-in-progress.
It is a fundamental part of QRM (Quality Risk Management) to understand the process application and risk to product exposure. It is the air flow patterns that determine the contamination removal effectiveness of particles released from equipment and personnel (both viable and non-viable).
In conclusion, the take home message from this blog is that it’s all about airflow patterns and contamination removal effectiveness, not “rule of thumb” air change rates.